Case C-315/24 Nestlé Sverige AB v Miljönämnden i Helsingborgs kommun
An introduction, background information and details of the Court of Justice of the European Union (CJEU) ruling made on 09 October 2025 are provided in the FSAI news article.
Clarification on the scope of the court ruling
The court ruling confirms that no repetition of nutrition information on Foods for Special Medical Purposes is permitted. This means that any reference to the product's energy value and/or the amounts of various nutrients provided anywhere on the label other than in the nutrition declaration is not permitted.
Regulation 1169/2011 on the provision of food information to the consumer (FIC) defines food information as “information concerning a food and made available to the final consumer by means of a label, other accompanying material, or any other means including modern technology tools or verbal communication”.
The prohibition of the repetition of nutrition information expands to the use of nutrition and health claims whereby Article 7 (Nutrition and Health Claims) of Delegated Regulation (EU) 2016/128 states “Nutrition and health claims shall not be made on food for special medical purposes”.
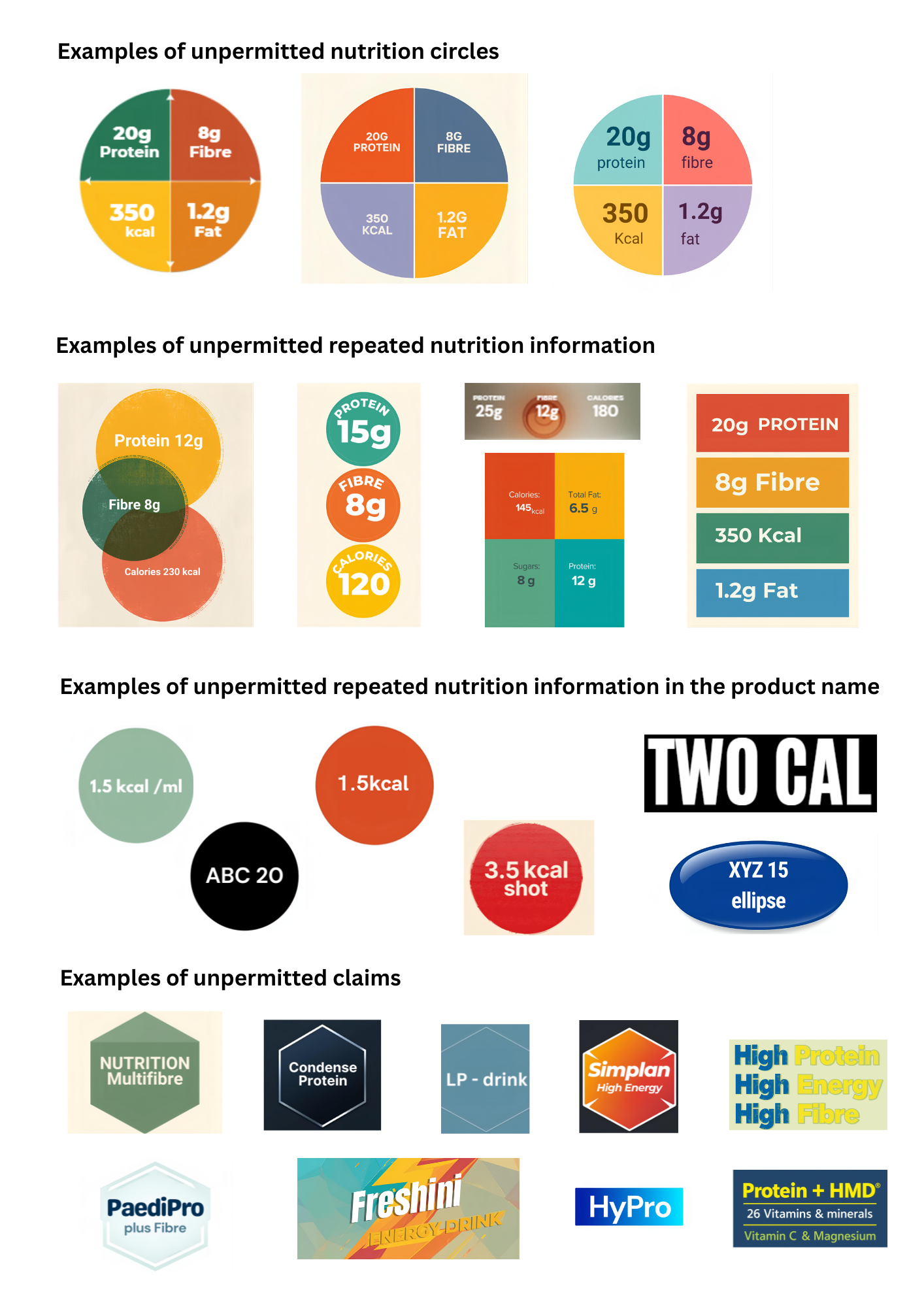
The scope of this ruling includes:
- The name of the food, for example ‘2Kcal’ drink is a reference to the nutrition information and is not permitted
- Words, pictures, numbers and graphics used to emphasise the nutrition information is not permitted. For example, the use of the number in the name of the food to reference part of its nutrition content for example XYZ 20 where 20 refers to the protein
- Repeating the nutrition information anywhere on the label through any means is not permitted. For example, a nutrition circle or highlighting certain nutrition information like the Kcal, protein, fat or fibre content
In addition, and complimentary to the ruling:
- Reference to nutrition and health claims are not permitted for example “High Energy”, “High protein”, “Multi fibre”, “Energy” or “Protein” and this extends to the name of the food as per Regulation 1924/2006, see examples
- Repetition of the nutrition information in the form of claims are not permitted in the product description required on FSMPs as per Article 5(2)(g) Regulation (EU) 2016/128, example:
- Instead of ‘High energy’ the following example is acceptable ‘For the dietary management of those with medically identified increased energy needs.’
- Instead of ‘High protein’ the following example is acceptable ‘For the dietary management of disease related malnutrition in patients with higher protein needs.’
The description should state how the product meets the dietary needs of the patient it is intended for, NOT claim the product is high in or simply contains a nutrient without context. The description must align with the rationale of the use of the product, for example:
- Instead of “High in energy and protein and contains vitamins and minerals” as it is too general and not specific to the needs of the patient.
- The following example is acceptable “For the dietary management of chronic wounds in patients with increased energy, protein, arginine and micronutrient needs.’
As per article 5 2(g) ‘a description’ refers to a single consolidated description of the product characteristics. It does not support the repetition of this description throughout the packaging for the purposes of marketing or advertising to consumers or healthcare professionals. The single description in combination with the nutrition declaration accompanying these products is intended to provide healthcare professionals with a comprehensive understanding of the nutritional composition enabling comparison between products. The description may be located on the front of pack, back of pack or side of pack, but should only appear once.
N.B. Where nutrients are adapted to meet the specific needs of a disease, condition or otherwise, scientific substantiation is required to be submitted with the notification to explain the rational for the adaption from ANNEX I of Regulation (EU) 2016/128.
Examples of unpermitted labelling on FSMPs**
.png?lang=en-IE)
Examples of unpermitted descriptions repeating nutrition information and containing claims
- High-energy, high protein nutrition with sugars and sweeteners
- High-protein high energy food for special medical purposes
- Energy dense 2.0Kcal/ml with 25g protein and 5g fibre per 250ml
- DHA supplement that contains vitamins, minerals, essential amino acids and trace elements
- High energy with specific micronutrients
- Complete nutrition with FOS
- Nutritionally complete milkshake with vitamins and minerals
**Please note the above are comparable examples of non-compliances identified on FSMPs produced by multiple FSMP manufacturers. These examples are not exhaustive and only offer guidance on what is non-compliant. The responsibility is on the food business to comply with the relevant legislation of the product category placed on the market.
